For a long time, I’ve wrestled with how to convince people of three very important things:
Climate change is real.
Humans are undeniably causing it.
We should (and can) do something about it.
As a coral biologist, these are no-brainers for me. Over half of the world’s coral reefs have disappeared since the 1950s due to rising ocean temperatures and human activities1. And almost all of what’s left is likely to be gone by 20502. But it doesn’t have to be this way. We still have time to protect what remains, and help reefs recover if we act now.
So when I talk about climate change, I’m not speaking in hypotheticals. I’m watching it happen right in front of me.
And yet, climate change has become one of the most politically charged topics of our time, despite the scientific consensus being remarkably strong3.
I’m here to set the record straight.
My goal is to write as many posts as it takes to help you understand everything you need to know about climate change.
I believe that knowledge is power. And if enough people have this knowledge, we will finally have the power to take back our future from those who would sacrifice it for short-term profit.
We can choose a better way forward.
But for now, let’s travel back to the early 19th century, when scientists first began asking a question that would eventually lead to one of the most important scientific discoveries of all time.
“What determines a planet’s temperature?”
In the 1820s, French physicist Joseph Fourier asked this deceptively simple question while studying Earth’s climate.
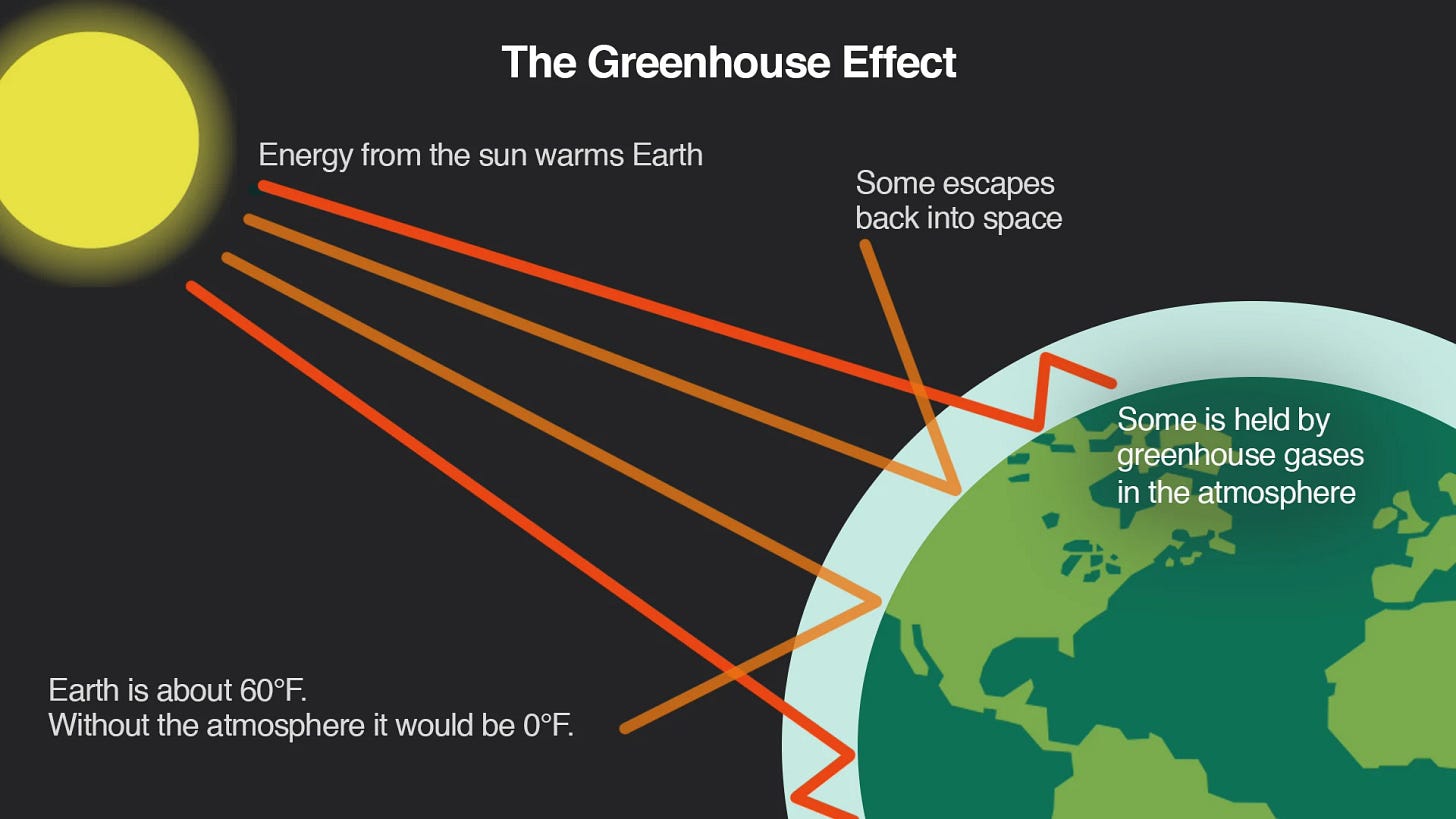
Based on Earth’s size and distance from the Sun, Fourier calculated that our planet should be much colder—a brisk 0˚F. So how could it be that Earth’s actual temperature was a much more comfortable 59˚F?
Something wasn’t adding up.
Fourier proposed that Earth’s atmosphere acts like a blanket, trapping heat from the Sun and slowing its escape back into space4. He didn’t know exactly how it worked, or which gases in the atmosphere were responsible, but he understood one thing very clearly:
The atmosphere plays a major role in regulating Earth’s temperature.
This idea became the foundation of what we now call the Greenhouse Effect.
A few decades later, Irish physicist John Tyndall took this idea to the laboratory.
In 1859, Tyndall built one of the first ratio spectrophotometers — a device that allowed him to measure how different gases absorb and transmit heat.
Through a series of experiments, he discovered something remarkable: invisible gases in our atmosphere behave very differently when it comes to trapping heat.5
Gases like oxygen, nitrogen, and hydrogen — the main components of our atmosphere — were almost completely transparent to infrared radiation (meaning they let heat go right through them without absorbing any).
Minor gases in the atmosphere, specifically water vapor, carbon dioxide, methane, and ozone, were exceptionally good at absorbing and trapping heat.
Even in small quantities, these gases absorbed far more heat than the bulk of the atmosphere itself.
For the first time, scientists had hard experimental evidence:
The composition of the atmosphere (not just the atmosphere itself) controls Earth’s temperature.
By the late 1800s, scientists understood that certain gases act like an invisible blanket around the planet—a phenomenon we now call the Greenhouse Effect.
Pause: What exactly is the Greenhouse Effect?
It’s probably the single most important process in all of climate science, so let’s interrupt our history lesson to talk about how it works:
Sunlight passes easily through the atmosphere and reaches Earth’s surface
Earth’s surface absorbs that sunlight and warms up (think hot pavement on a sunny day)
Earth doesn’t just hold onto all that energy from the sun. It tries to get rid of some of it by re-radiating it upwards as infrared radiation (which is basically energy in the form of heat)
Greenhouse gases like water vapor, carbon dioxide, and methane are mostly invisible to sunlight, but are very good at absorbing infrared radiation
They trap some of this outgoing heat and re-emit it in all directions, including back toward Earth’s surface
The result is that some of the heat that would have escaped into space instead stays in the atmosphere, warming the planet.
This is really similar to how a greenhouse works. Sunlight passes through the glass, the surfaces inside absorb the sunlight and warm up, and the heat tries to escape — but the glass traps most of it, keeping the greenhouse warmer than the air outside.
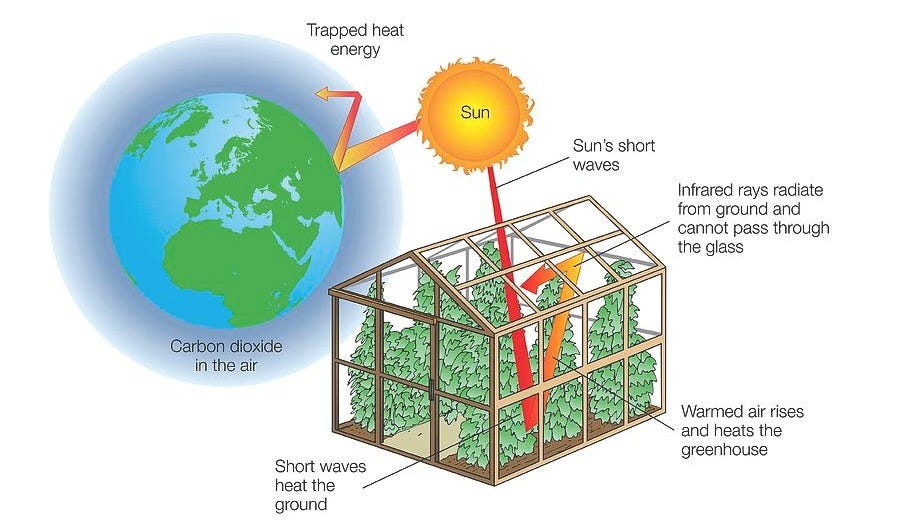
The same basic principle applies to Earth’s atmosphere, which is why we call it the Greenhouse Effect. And it’s not necessarily a bad thing! Without it, Earth would be a barren, frozen rock, much too cold to support life.
But I urge you to pause here and form your own hypothesis: Given what we know about greenhouse gases, what do you think could happen if the amount of these greenhouse gases in the atmosphere were to increase or decrease? I recommend that you spend a moment thinking about this on your own before continuing.
Okay, now back to history.
About 40 years after Tyndall’s experiments, Swedish chemist Svante Arrhenius took it one step further.
He wanted to understand what could drive major changes in Earth’s climate — like the ones that triggered the ice ages.
Arrhenius knew about Fourier’s and Tyndall’s work, and he began to suspect that changes in the amount of these heat-trapping gases (especially carbon dioxide) could have a significant effect on Earth’s temperature. So he set out to test this idea mathematically.
In 1896, he published a groundbreaking hypothesis6:
A doubling of atmospheric CO₂ would result in approximately a 5–6°C increase in global average temperature.
Arrhenius wasn’t trying to sound the alarm about environmental disaster. He was applying the basic principles of physics and chemistry to answer one of the greatest scientific mysteries of his time.
Building on the work of Fourier and Tyndall, Arrhenius was the first to quantify just how much the planet could warm (or cool) based on changing levels of greenhouse gases.
Now, his estimates weren’t perfect. Modern climate models, which account for a wider range of variables, suggest a likely increase of about 3°C for a doubling of CO₂.
But still, it was a revolutionary discovery:
Earth’s climate isn’t fixed.
It could change depending on the amount of CO2 in the atmosphere.
For decades, Arrhenius’ work sat largely unnoticed. But by the mid-20th century, that started to change.
In 1958, an American scientist named Charles David Keeling began doing something no one had ever done before: he started measuring the actual concentration of carbon dioxide in the atmosphere on a continuous basis.
He set up instruments high on the Mauna Loa volcano in Hawaii, far from local pollution sources, to get the clearest reading possible of global atmospheric CO₂.
The result became one of the most important datasets in all of climate science: the Keeling Curve.
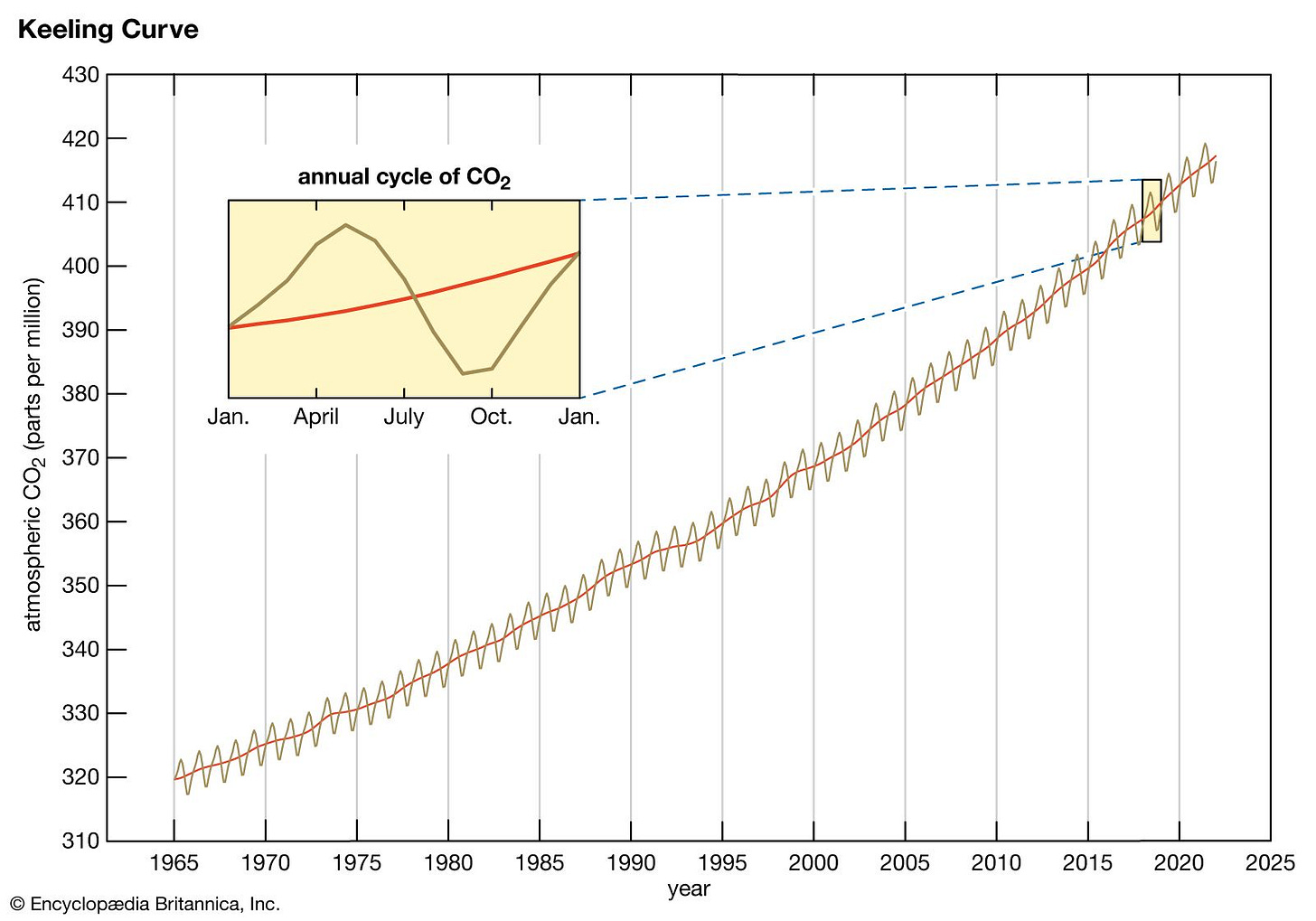
What it showed was simple and undeniable:
CO₂ levels are rising.
Not slowly. Not randomly. But steadily, year after year. Why?

We’re pumping it straight into the atmosphere.
And in many ways, we’re doing it through the largest unintended experiment in human history.
So if you take away one thing from this post, let it be this:
Earth is getting hotter because CO₂ levels are rising.
That’s it. That’s the core mechanism.
It was predicted over a century ago.
It’s been measured and tested and validated by different scientists with different backgrounds for more than half a century.
This isn’t politics. This is physics.
And understanding it is the first step toward changing the trajectory we’re on.
Eddy, Tyler D. et al. (2021). Global decline in capacity of coral reefs to provide ecosystem services. One Earth, 4(9), 1278-1285. https://doi.org/10.1016/j.oneear.2021.08.016.
Souter, D. et al. (2020). Status of Coral Reefs of the World: 2020. https://gcrmn.net/wp-content/uploads/2022/05/Status-of-Coral-Reefs-of-the-World-2020-Summary-for-Policymakers.pdf
It’s important to note that scientists focus on evidence, not on opinions.
NASA. (2024, October 21). Scientific Consensus - NASA Science. NASA. https://science.nasa.gov/climate-change/scientific-consensus/
Fleming, J. R. (2005). Joseph Fourier’s Theory of Terrestrial Temperatures. In Historical Perspectives on Climate Change. Oxford University Press.
Tyndall, J. (1860). VII. Note on the transmission of radiant heat through gaseous bodies. Proceedings of the Royal Society of London. (10), 37-39. http://doi.org/10.1098/rspl.1859.0017
Arrhenius, S. (1896). On the influence of carbonic acid in the air upon the temperature of the ground. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 41(251), 237-276.







